Chemical compound
Pharmaceutical compound
Dichloropane ATC code Legal status
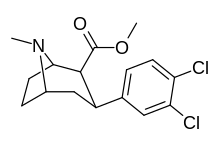
Methyl (1R ,2S ,3S ,5S )-3-(3,4-dichlorophenyl)-8-azabicyclo[3.2.1]octane-2-carboxylate
CAS Number PubChem CID ChemSpider UNII CompTox Dashboard (EPA ) Formula C 15 H 17 Cl 2 N O 2 Molar mass −1 3D model (JSmol )
COC(=O)[C@@H]1[C@H]2CC[C@H](N2C)C[C@@H]1C3=CC(=C(C=C3)Cl)Cl
InChI=1S/C16H19Cl2NO2/c1-19-10-4-6-14(19)15(16(20)21-2)11(8-10)9-3-5-12(17)13(18)7-9/h3,5,7,10-11,14-15H,4,6,8H2,1-2H3/t10-,11+,14+,15-/m0/s1
N Key:JFUNLJPTPCQLIR-PJQZNRQZSA-N
N N Y (what is this?) (verify)
Dichloropane ((−)-2β-Carbomethoxy-3β-(3,4-dichlorophenyl)tropane , RTI-111 , O-401 ) is a stimulant of the phenyltropane class that acts as a serotonin–norepinephrine–dopamine reuptake inhibitor (SNDRI) with IC50 values of 3.13, 18, and 0.79 nM, respectively.[ 1] cocaine .[ 2] [ 3]
Methylecgonidine is the direct precursor to this compound.[ 4]
Trans -CO2 Me group
The thermodynamic isomer with a trans -CO2 Me group is still active. This isomer was used by Neurosearch to make three different phenyltropanes which were tested in clinical trials.
See also
References
^ Carroll FI, Blough BE, Nie Z, Kuhar MJ, Howell LL, Navarro HA (April 2005). "Synthesis and monoamine transporter binding properties of 3beta-(3',4'-disubstituted phenyl)tropane-2beta-carboxylic acid methyl esters". Journal of Medicinal Chemistry . 48 (8): 2767–71. doi :10.1021/jm040185a . PMID 15828814 . ^ Ranaldi R, Anderson KG, Carroll FI, Woolverton WL (December 2000). "Reinforcing and discriminative stimulus effects of RTI 111, a 3-phenyltropane analog, in rhesus monkeys: interaction with methamphetamine". Psychopharmacology . 153 (1): 103–10. doi :10.1007/s002130000602 . PMID 11255920 . S2CID 29716872 . ^ Cook CD, Carroll IF, Beardsley PM (December 2001). "Cocaine-like discriminative stimulus effects of novel cocaine and 3-phenyltropane analogs in the rat". Psychopharmacology . 159 (1): 58–63. doi :10.1007/s002130100891 . PMID 11797070 . S2CID 25696981 . ^ Carroll FI, Mascarella SW, Kuzemko MA, Gao Y, Abraham P, Lewin AH, et al. (September 1994). "Synthesis, ligand binding, and QSAR (CoMFA and classical) study of 3 beta-(3'-substituted phenyl)-, 3 beta-(4'-substituted phenyl)-, and 3 beta-(3',4'-disubstituted phenyl)tropane-2 beta-carboxylic acid methyl esters". Journal of Medicinal Chemistry . 37 (18): 2865–73. doi :10.1021/jm00044a007 . PMID 8071935 .
DAT Tooltip Dopamine transporter (DRIs Tooltip Dopamine reuptake inhibitors )
NET Tooltip Norepinephrine transporter (NRIs Tooltip Norepinephrine reuptake inhibitors )
Others: Antihistamines (e.g., brompheniramine , chlorphenamine , pheniramine , tripelennamine )Antipsychotics (e.g., loxapine , ziprasidone )Arylcyclohexylamines (e.g., ketamine , phencyclidine )Dopexamine Ephenidine Ginkgo biloba Indeloxazine Nefazodone Opioids (e.g., desmetramadol , methadone , pethidine (meperidine) , tapentadol , tramadol , levorphanol )
SERT Tooltip Serotonin transporter (SRIs Tooltip Serotonin reuptake inhibitors )
Others: A-80426 Amoxapine Antihistamines (e.g., brompheniramine , chlorphenamine , dimenhydrinate , diphenhydramine , mepyramine (pyrilamine) , pheniramine , tripelennamine )Antipsychotics (e.g., loxapine , ziprasidone )Arylcyclohexylamines (e.g., 3-MeO-PCP , esketamine , ketamine , methoxetamine , phencyclidine )Cyclobenzaprine Delucemine Dextromethorphan Dextrorphan Efavirenz Hypidone Medifoxamine Mesembrine Mifepristone MIN-117 (WF-516) N-Me-5-HT Opioids (e.g., dextropropoxyphene , methadone , pethidine (meperidine) , levorphanol , tapentadol , tramadol )Roxindole
VMATs Tooltip Vesicular monoamine transporters Others
2-Carboxymethyl Esters (3,4-Disubstituted Phenyl)-tropanes Arylcarboxy Carboxyalkyl Acyl β,α Stereochemistry α,β Stereochemistry Heterocycles: 3-Substituted-isoxazol-5-yl Heterocycles: 3-Substituted-1,2,4-oxadiazole N-alkyl N-replaced (S,O,C) Irreversible Nortropanes (N-demethylated)