|
Fenclonine
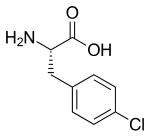
Fenclonine, also known as para-chlorophenylalanine (PCPA), acts as a selective and irreversible inhibitor of tryptophan hydroxylase, which is a rate-limiting enzyme in the biosynthesis of serotonin.[2] It has been used experimentally to treat carcinoid syndrome, but the side effects, mostly hypersensitivity reactions and psychiatric disturbances, have prevented development for this use.[3] The effects of serotonin depletion from fenclonine are so drastic that serotonin cannot even be detected immunohistochemically within the first day after administration of a control dose. Tryptophan hydroxylase activity can be detected neither in cell bodies or nerve terminals. After one week 10% of control values (the baseline extrapolated for the study) had replenished in the raphe nucleus, and after two weeks from initial treatment as much was again detected in the hypothalamus region. Aromatic L-amino acid decarboxylase (AADC) levels were at no time affected.[4] It is used in scientific research in humans[5] and animals[2] to investigate the effects of serotonin depletion. See also
References
|
||||||||||||||||||||||||||||||||||||||||||||||||