|
Hydrohalite
Hydrohalite is a mineral that occurs in saturated halite brines at cold temperatures (below 0.1 °C). It was first described in 1847 in Dürrnberg, Austria. It exists in cold weather. 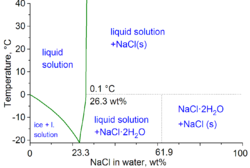 Hydrohalite has a high nucleation energy, and solutions will normally need to be supercooled for crystals to form. The cryohydric point is at −21.2 °C (−6.2 °F). Above this temperature, liquid water saturated with salt can exist in equilibrium with hydrohalite. Hydrohalite has a strong positive temperature coefficient of solubility, unlike halite.[2] Hydrohalite decomposes at 0.1°C, giving a salty brine and solid halite. Under pressure, hydrohalite is stable between 7,900 and 11,600 atmospheres pressure. The decomposition point increases at the rate of 0.007K per atmosphere (for 1–1000 atmospheres).[2] The maximum decomposition temperature is at 25.8°C under 9400 atmospheres. Above this pressure the decomposition point goes down.[2] CeresHydrohalite was discovered on Ceres by Dawn,[3] suggesting an early ocean, possibly surviving as a relict ocean. References
|
||||||||||||||||||||||||