Chemical compound
Lutetium(III) iodide or lutetium iodide is an inorganic compound consisting of iodine and lutetium , with the chemical formula of LuI3 .
Preparation
Lutetium(III) iodide can be obtained by reacting lutetium with iodine :[ 5] [ 2]
2 Lu + 3 I2 → LuI3 Lutetium(III) iodide can also obtained by the reacting metallic lutetium with mercury iodide in vacuum at 500 °C:[ 5]
2 Lu + 3 HgI2 → 2 LuI3 + 3 Hg The elemental mercury generated in the reaction can be removed by distillation .[ 6]
The lutetium(III) iodide hydrate crystallized from the solution can be heated with ammonium iodide to obtain the anhydrate .[ 7] [ 5]
Properties
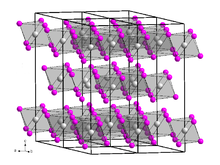
It is a brown, very hygroscopic solid with a bismuth(III) iodide -type crystal structure . In air, it quickly absorbs moisture and forms hydrates .[ 2] [ 3] [ 4] [ 5]
Lutetium(III) iodide doped with cerium is designed for use in PET scanners .[ 8] yttrium iodide and gadolinium iodide in LuI3 -YI3 -GdI3 scintillators to detect neutron and gamma radiation .[ 9]
References
^ a b c d e Lutetium(III) iodide, ultra dry, 99.9% (REO) at AlfaAesar, accessed on {{{Datum}}} (PDF ) (JavaScript required).[dead link ] ^ a b c Webelements: Lutetium: lutetium triiodide Retrieved 31.3.2018^ a b Americanelements.com: Lutetium Iodide Retrieved 31.3.2018^ a b
Perry, Dale L. (2016). Handbook of Inorganic Compounds ISBN 9781439814628 . Retrieved 2018-03-31 .
^ a b c d e Georg Brauer (Hrsg.), unter Mitarbeit von Marianne Baudler u. a.: Handbuch der Präparativen Anorganischen Chemie. 3., umgearbeitete Auflage. Band I, Ferdinand Enke, Stuttgart 1975, ISBN 3-432-02328-6, S. 1077.^ Asprey, L. B.; Keenan, T. K.; Kruse, F. H. Preparation and crystal data for lanthanide and actinide triiodides. Inorg. Chem., 1964. 3 (8): 1137-1240
^ 无机化学丛书 第七卷 钪 稀土元素. 科学出版社. pp 211
^ Saha, Gopal B. (2010). Basics of PET Imaging: Physics, Chemistry, and Regulations ISBN 9781441908056 . Retrieved 2018-03-31 . ^ Glodo, Jarek; van Loef, Edgar V. D. & Higgins, William M. (2008-06-17). "Mixed Lutetium Iodide Compounds". IEEE Transactions on Nuclear Science . 55 (3): 1496–1500. Bibcode :2008ITNS...55.1496G . doi :10.1109/TNS.2008.922215 . ISSN 1558-1578 . S2CID 36257295 . {{cite journal }}: CS1 maint: multiple names: authors list (link )
Salts and covalent derivatives of the
iodide ion