|
Parkinson's disease
Parkinson's disease (PD), or simply Parkinson's, is a neurodegenerative disease primarily of the central nervous system, affecting both motor and non-motor systems. Symptoms typically develop gradually, with non-motor issues becoming more prevalent as the disease progresses. Common motor symptoms include tremors, bradykinesia (slowness of movement), rigidity, and balance difficulties, collectively termed parkinsonism. In later stages, Parkinson's disease dementia, falls, and neuropsychiatric problems such as sleep abnormalities, psychosis, mood swings, or behavioral changes may arise. Most cases of Parkinson's disease are sporadic, though contributing factors have been identified. Pathophysiology involves progressive degeneration of nerve cells in the substantia nigra, a midbrain region that provides dopamine to the basal ganglia, a system involved in voluntary motor control. The cause of this cell death is poorly understood but involves the aggregation of alpha-synuclein into Lewy bodies within neurons. Other potential factors involve genetic and environmental influences, medications, lifestyle, and prior health conditions. Diagnosis is primarily based on signs and symptoms, typically motor-related, identified through neurological examination. Medical imaging techniques like positron emission tomography can support the diagnosis. Parkinson's typically manifests in individuals over 60, with about one percent affected. In those younger than 50, it is termed "early-onset PD". No cure for Parkinson's is known, and treatment focuses on alleviating symptoms. Initial treatment typically includes L-DOPA, MAO-B inhibitors, or dopamine agonists. As the disease progresses, these medications become less effective and may cause involuntary muscle movements. Diet and rehabilitation therapies can help improve symptoms. Deep brain stimulation is used to manage severe motor symptoms when drugs are ineffective. There is little evidence for treatments addressing non-motor symptoms, such as sleep disturbances and mood instability. Life expectancy for those with PD is near-normal but is decreased for early-onset. Classification and terminologyParkinson's disease (PD) is a neurodegenerative disease affecting both the central and peripheral nervous systems, characterized by the loss of dopamine-producing neurons in the substantia nigra region of the brain.[5] It is classified as a synucleinopathy due to the abnormal accumulation of the protein alpha-synuclein, which aggregates into Lewy bodies within affected neurons.[6] The loss of dopamine-producing neurons in the substantia nigra initially presents as movement abnormalities, leading to Parkinson's further categorization as a movement disorder.[1] In 30% of cases, disease progression leads to the cognitive decline known as Parkinson's disease dementia (PDD).[7] Alongside dementia with Lewy bodies, PDD is one of the two subtypes of Lewy body dementia.[8] The four cardinal motor symptoms of Parkinson's—bradykinesia (slowed movements), postural instability, rigidity, and tremor—are called parkinsonism.[9][10] These four symptoms are not exclusive to Parkinson's and can occur in many other conditions,[11][12] including HIV infection and recreational drug use.[13][14] Neurodegenerative diseases that feature parkinsonism but have distinct differences are grouped under the umbrella of Parkinson-plus syndromes or, alternatively, atypical parkinsonian disorders.[15][16] Parkinson's disease can be attributed to genetic factors or be idiopathic, in which there is no clearly identifiable cause. The latter, also called sporadic Parkinson's, makes up some 85–90% of cases.[17] Signs and symptomsMotorMotor symptoms include a stooping posture, the "Parkinsonian gait", and jagged, diminutive handwriting. Although a wide spectrum of motor and non-motor symptoms appear in Parkinson's, the cardinal features remain tremor, bradykinesia, rigidity, and postural instability, collectively termed parkinsonism.[18] Appearing in 70–75 percent of PD patients,[18][19] tremor is often the predominant motor symptom.[18] Resting tremor is the most common, but kinetic tremors—occurring during voluntary movements—and postural tremor—preventing upright, stable posture—also occur.[19] Tremor largely affects the hands and feet:[19] a classic parkinsonian tremor is "pill-rolling", a resting tremor in which the thumb and index finger make contact in a circular motion at 4–6 Hz frequency.[20][21] Bradykinesia describes difficulties in motor planning, beginning, and executing, resulting in overall slowed movement with reduced amplitude that affects sequential and simultaneous tasks.[22] Bradykinesia can also lead to hypomimia, reduced facial expressions.[21] Rigidity, also called rigor, refers to a feeling of stiffness and resistance to passive stretching of muscles that occurs in up to 89 percent of cases.[23][24] Postural instability typically appears in later stages, leading to impaired balance and falls.[25] Postural instability also leads to a forward stooping posture.[26] Beyond the cardinal four, other motor deficits, termed secondary motor symptoms, commonly occur.[27] Notably, gait disturbances result in the Parkinsonian gait, which includes shuffling and paroxysmal deficits, where a normal gait is interrupted by rapid footsteps—known as festination—or sudden stops, impairing balance and causing falls.[28] [29] Most PD patients experience speech problems, including stuttering, hypophonic, "soft" speech, slurring, and festinating speech (rapid and poorly intelligible).[30] Handwriting is commonly altered in Parkinson's, decreasing in size—known as micrographia—and becoming jagged and sharply fluctuating.[31] Grip and dexterity are also impaired.[32] Non-motorNeuropsychiatric and cognitive
Neuropsychiatric symptoms like anxiety, apathy, depression, hallucinations, and impulse control disorders occur in up to 60% of those with Parkinson's. They often precede motor symptoms and vary with disease progression.[34] Non-motor fluctuations, including dysphoria, fatigue, and slowness of thought, are also common.[35] Some neuropsychiatric symptoms are not directly caused by neurodegeneration but rather by its pharmacological management.[36] Cognitive impairments rank among the most prevalent and debilitating non-motor symptoms.[37] These deficits may emerge in the early stages or before diagnosis,[37][38] and their prevalence and severity tend to increase with disease progression. Ranging from mild cognitive impairment to severe Parkinson's disease dementia, these impairments include executive dysfunction, slowed cognitive processing speed, and disruptions in time perception and estimation.[38] Autonomic Autonomic nervous system failures, known as dysautonomia, can appear at any stage of Parkinson's.[39][40] They are among the most debilitating symptoms and greatly reduce quality of life.[41] Although almost all PD patients suffer cardiovascular autonomic dysfunction, only some are symptomatic.[41] Chiefly, orthostatic hypotension—a sustained blood pressure drop of at least 20 mmHg systolic or 10 mmHg diastolic after standing—occurs in 30–50 percent of cases. This can result in lightheadedness or fainting: subsequent falls are associated with higher morbidity and mortality.[41][42] Other autonomic failures include gastrointestinal issues like chronic constipation, impaired stomach emptying and subsequent nausea, excessive salivation, and dysphagia (difficulty swallowing): all greatly reduce quality of life.[43][44] Dysphagia, for instance, can prevent pill swallowing and lead to aspiration pneumonia.[45] Urinary incontinence, sexual dysfunction, and thermoregulatory dysfunction—including heat and cold intolerance and excessive sweating—also frequently occur.[46] Other non-motor symptomsSensory deficits appear in up to 90 percent of patients and are usually present at early stages.[47] Nociceptive and neuropathic pain are common,[47] with peripheral neuropathy affecting up to 55 percent of individuals.[48] Visual impairments are also frequently observed, including deficits in visual acuity, color vision, eye coordination, and visual hallucinations.[49] An impaired sense of smell is also prevalent.[50] PD patients often struggle with spatial awareness, recognizing faces and emotions, and may experience challenges with reading and double vision.[51] Sleep disorders are highly prevalent in PD, affecting up to 98%.[52] These disorders include insomnia, excessive daytime sleepiness, restless legs syndrome, REM sleep behavior disorder (RBD), and sleep-disordered breathing, many of which can be worsened by medication. RBD may begin years before the initial motor symptoms. Individual presentation of symptoms varies, although most people affected by PD show an altered circadian rhythm at some point of disease progression.[53][54] PD is also associated with a variety of skin disorders that include melanoma, seborrheic dermatitis, bullous pemphigoid, and rosacea.[55] Seborrheic dermatitis is recognized as a premotor feature that indicates dysautonomia and demonstrates that PD can be detected not only by changes of nervous tissue, but tissue abnormalities outside the nervous system as well.[56] CausesThe protein alpha-synuclein aggregates into Lewy bodies and neurites, stained brown at right. As of 2024, the cause of neurodegeneration in Parkinson's remains unclear,[57] though it is believed to result from the interplay of genetic and environmental factors.[57] The majority of cases are sporadic with no clearly identifiable cause, while approximately 5–10 percent are familial.[58] Around a third of familial cases can be attributed to a single monogenic cause.[58] Molecularly, abnormal aggregation of alpha-synuclein is considered a key contributor to PD pathogenesis,[57] although the trigger for this aggregation remains debated.[59] Proteostasis disruption and the dysfunction of cell organelles, including endosomes, lysosomes, and mitochondria, are implicated in pathogenesis.[57][60] Additionally, maladaptive immune and inflammatory responses are potential contributors.[57] The substantial heterogeneity in PD presentation and progression suggests the involvement of multiple interacting triggers and pathogenic pathways.[59] Genetic Parkinson's can be narrowly defined as a genetic disease, as rare inherited gene variants have been firmly linked to monogenic PD, and the majority of sporadic cases carry variants that increase PD risk.[57][61][62] PD heritability is estimated to range from 22 to 40 percent.[57] Around 15 percent of diagnosed individuals have a family history, of which 5–10 percent can be attributed to a causative risk gene mutation. However, carrying one of these mutations may not lead to disease. Rates of familial PD vary by ethnicity: monogenic PD occurs in up to 40% of Arab-Berber patients and 20% of Ashkenazi Jewish patients.[62] As of 2024, around 90 genetic risk variants across 78 genomic loci have been identified.[63] Notable risk variants include SNCA (which encodes alpha-synuclein), LRRK2, and VPS35 for autosomal dominant inheritance, and PRKN, PINK1, and DJ1 for autosomal recessive inheritance.[57][64] LRRK2 is the most common autosomal dominant variant, responsible for 1–2 percent of all PD cases and 40 percent of familial cases.[65] [58] Parkin variants are associated with nearly half of recessive, early-onset monogenic PD.[66] Mutations in the GBA1 gene, linked to Gaucher's disease, are found in 5–15 percent of PD cases.[67] The GBA1 variant frequently leads to cognitive decline.[65] Environmental The limited heritability of Parkinson's strongly suggests environmental factors are involved, though identifying these risk factors and establishing causality is challenging due to PD's decade-long prodromal period.[68] However, environmental toxicants such as air pollution, pesticides, and industrial solvents like trichloroethylene are strongly linked to Parkinson's.[69] Certain pesticides—like paraquat, glyphosate, and rotenone—are the most established environmental toxicants for Parkinson's and are likely causal.[70][71][72] PD prevalence is strongly associated with local pesticide use, and many pesticides are mitochondrial toxins.[73] Paraquat, for instance, structurally resembles metabolized MPTP,[70] which selectively kills dopaminergic neurons by inhibiting mitochondrial complex 1 and is widely used to model PD.[74][70] Pesticide exposure after diagnosis may also accelerate disease progression.[70] Without pesticide exposure, an estimated 20 percent of all PD cases would be prevented.[75] HypothesesPrionic hypothesisThe hallmark of Parkinson's is the formation of protein aggregates, beginning with alpha-synuclein fibrils and followed by Lewy bodies and Lewy neurites.[76] The prion hypothesis suggests that alpha-synuclein aggregates are pathogenic and can spread to neighboring, healthy neurons and seed new aggregates. Some propose that the heterogeneity of PD may stem from different "strains" of alpha-synuclein aggregates and varying anatomical sites of origin.[77][78] Alpha-synuclein propagation has been demonstrated in cell and animal models and is the most popular explanation for the progressive spread through specific neuronal systems.[79] However, therapeutic efforts to clear alpha-synuclein have failed.[80] Additionally, postmortem brain tissue analysis shows that alpha-synuclein pathology does not clearly progress through the nearest neural connections.[81] Braak's hypothesisIn 2002, Heiko Braak and colleagues proposed that Parkinson's disease begins outside the brain and is triggered by a "neuroinvasion" of some unknown pathogen.[82][83] The pathogen enters through the nasal cavity and is swallowed into the digestive tract, initiating Lewy pathology in both areas.[72][82] This alpha-synuclein pathology may then travel from the gut to the central nervous system through the vagus nerve.[84] This theory could explain the presence of Lewy pathology in both the enteric nervous system and olfactory tract neurons, as well as clinical symptoms like loss of small and gastrointestinal problems.[83] It has also been suggested that environmental toxicants might be ingested in a similar manner to trigger PD.[85] Catecholaldehyde hypothesis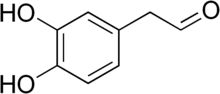 The enzyme monoamine oxidase (MAO) plays a central role in the metabolism of the neurotransmitter dopamine and other catecholamines. The catecholaldehyde hypothesis argues that the oxidation of dopamine by MAO into 3,4-dihydroxyphenylacetaldehyde (DOPAL) and hydrogen peroxide and the subsequent abnormal accumulation thereof leads to neurodegeneration. The theory posits that DOPAL interacts with alpha-synuclein and causes it to aggregate.[86][87] Mitochondrial dysfunctionWhether mitochondrial dysfunction is a cause or consequence of PD pathology remains unclear.[88] Impaired ATP production, increased oxidative stress, and reduced calcium buffering may contribute to neurodegeneration.[89] The finding that MPP+—a respiratory complex I inhibitor and MPTP metabolite—caused parkinsonian symptoms strongly implied that mitochondria contributed to PD pathogenesis.[90][91] Alpha-synuclein and toxicants like rotenone similarly disrupt respiratory complex I.[92] Additionally, faulty gene variants involved in familial Parkinson's—including PINK1 and Parkin—prevent the elimination of dysfunctional mitochondria through mitophagy.[93][94] NeuroinflammationSome hypothesize that neurodegeneration arises from a chronic neuroinflammatory state created by local activated microglia and infiltrating immune cells.[57] Mitochondrial dysfunction may also drive immune activation, particularly in monogenic PD.[57] Some autoimmune disorders increase the risk of developing PD, supporting an autoimmune contribution.[95] Additionally, influenza and herpes simplex virus infections increase the risk of PD, possibly due to a viral protein resembling alpha-synuclein.[96] Parkinson's risk is also decreased with immunosuppressants.[57] Pathophysiology Parkinson's disease has two hallmark pathophysiological processes: the abnormal aggregation of alpha-synuclein that leads to Lewy pathology, and the degeneration of dopaminergic neurons in the substantia nigra pars compacta.[97][98] The death of these neurons reduces available dopamine in the striatum, which in turn affects circuits controlling movement in the basal ganglia.[98] By the time motor symptoms appear, 50–80 percent of all dopaminergic neurons in the substantia nigra have degenerated.[98] However, cell death and Lewy pathology are not limited to the substantia nigra.[99] The six-stage Braak system holds that alpha-synuclein pathology begins in the olfactory bulb or outside the central nervous system in the enteric nervous system before ascending the brain stem.[100] In the third Braak stage, Lewy body pathology appears in the substantia nigra,[100] and, by the sixth step, Lewy pathology has spread to the limbic and neocortical regions.[101] Although Braak staging offers a strong basis for PD progression, the Lewy pathology around 50 percent patients do not adhere to the predicted model.[102] Indeed, Lewy pathology is highly variable and may be entirely absent in some PD patients.[99][103] Alpha-synuclein pathology Alpha-synuclein is an intracellular protein typically localized to presynaptic terminals and involved in synaptic vesicle trafficking, intracellular transport, and neurotransmitter release.[102][104] When misfolded, it can aggregate into oligomers and proto-fibrils that in turn lead to Lewy body formation.[104][105][106] Due to their lower molecular weight, oligomers and proto-fibrils may disseminate and be transmitted to other cells more rapidly.[106] Lewy bodies consist of a fibrillar exterior and granular core. Although alpha-synuclein is the dominant proteinaceous component, the core contains mitochondrial and autophagosomal membrane components, suggesting a link with organelle dysfunction.[107][108] It is unclear whether Lewy bodies themselves contribute to or are simply the result of PD pathogenesis: alpha-synuclein oligomers can independently mediate cell damage, and neurodegeneration can precede Lewy body formation.[109] Pathways involved in neurodegenerationThree major pathways—vesicular trafficking, lysosomal degradation, and mitochondrial maintenance—are known to be affected by and contribute to Parkinson's pathogenesis, with all three linked to alpha-synuclein.[110] High risk gene variants also impair all three of these processes.[110] All steps of vesicular trafficking are impaired by alpha-synuclein. It blocks endoplasmic reticulum (ER) vesicles from reaching the Golgi—leading to ER stress—and Golgi vesicles from reaching the lysosome, preventing alpha-synuclein degradation and leading to its build-up.[111] Risky gene variants, chiefly GBA, further compromise lysosomal function.[112] Although the mechanism is not well established, alpha-synuclein can impair mitochondrial function and cause subsequent oxidative stress. Mitochondrial dysfunction can in turn lead to further alpha-synuclein accumulation in a positive feedback loop.[113] Microglial activation, possibly caused by alpha-synuclein, is also strongly indicated.[114][115] Risk factorsPositive risk factorsAs 90 percent of Parkinson's cases are sporadic, the identification of the risk factors that may influence disease progression or severity is critical.[116][68] The most significant risk factor in developing PD is age, with a prevalence of 1 percent in those aged over 65 and approximately 4.3 percent in age over 85.[117] Traumatic brain injury significant increases PD risk, especially if recent.[118][119] Dairy consumption correlates with a higher risk, possibly due to contaminants like heptachlor epoxide.[120] Although the connection is unclear, melanoma diagnosis is associated with an approximately 45 percent risk increase.[120] There is also an association between methamphetamine use and PD risk.[120] Protective factors Although no compounds or activities have been mechanistically established as neuroprotective for Parkinson's,[121][122] several factors have been found to be associated with a decreased risk.[121] Tobacco use and smoking is strongly associated with a decreased risk, reducing the chance of developing PD by up to 70%.[123][124][120] Various tobacco and smoke components have been hypothesized to be neuroprotective, including nicotine, carbon monoxide, and monoamine oxidase B inhibitors.[125][126] Consumption of coffee, tea, or caffeine is also strongly associated with neuroprotection.[127][128] Prescribed adrenergic antagonists like terazosin may reduce risk.[127] Although findings have varied, usage of nonsteroidal anti-inflammatory drugs (NSAIDs) like ibuprofen may be neuroprotective.[129][130] Calcium channel blockers may also have a protective effect, with a 22% risk reduction reported.[131] Higher blood concentrations of urate—a potent antioxidant—have been proposed to be neuroprotective.[125][132] Although longitudinal studies observe a slight decrease in PD risk among those who consume alcohol—possibly due to alcohol's urate-increasing effect—alcohol abuse may increase risk.[133][134] DiagnosisDiagnosis of Parkinson's disease is largely clinical, relying on medical history and examination of symptoms, with an emphasis on symptoms that appear in later stages.[135][136] Although early stage diagnosis is not reliable,[136][137] prodromal diagnosis may consider previous family history of Parkinson's and possible early symptoms like rapid eye movement sleep behavior disorder (RBD), reduced sense of smell, and gastrointestinal issues.[138] Isolated RBD is a particularly significant sign as 90% of those affected will develop some form of neurodegenerative parkinsonism.[139] Diagnosis in later stages requires the manifestation of parkinsonism, specifically bradykinesia and rigidity or tremor. Further support includes other motor and non-motor symptoms and genetic profiling.[140] A PD diagnosis is typically confirmed by two of the following criteria: responsiveness to levodopa, resting tremor, levodopa-induced dyskinesia, or with dopamine transporter single-proton emission computed tomography.[140] If these criteria are not met, atypical parkinsonism is considered.[138] However, definitive diagnoses can only be made post-mortem through pathological analysis.[136] Misdiagnosis is common, with a reported error rate of near 25 percent, and diagnoses often change during follow-ups.[136][141] Diagnosis can be further complicated by multiple overlapping conditions.[136] Imaging Diagnosis can be aided by molecular imaging techniques such as magnetic resonance imaging (MRI), positron emission tomography (PET), and single-photon emission computed tomography (SPECT).[142] As both conventional MRI and computed tomography (CT) scans are usually normal in patients with early PD, they can be used to exclude other pathologies that cause parkinsonism.[141][143] Diffusion MRI can differentiate PD from multiple systems atrophy (MSA).[144] Emerging MRI techniques of at least 3.0 T field strength—including neuromelanin-MRI, 1H-MRSI, and resting state fMRI—may detect abnormalities in the substantia nigra, nigrostriatal pathway, and elsewhere.[141] Unlike MRI, PET and SPECT use radioisotopes for imaging.[145] Both techniques can aid diagnosis by characterizing PD-associated alterations in the metabolism and transport of dopamine in the basal ganglia.[146][147] Largely used outside the United States, iodine-123-meta-iodobenzylguanidine myocardial scintigraphy can assess heart muscle denervation to support a PD diagnosis.[148] Differential diagnosis Differential diagnosis of Parkinson's is among the most difficult in neurology.[149] Differentiating early PD from atypical parkinsonian disorders is a major difficulty. In their initial stages, PD can be difficult to distinguish from the atypical neurodegenerative parkinsonisms, including MSA, dementia with Lewy bodies, and the tauopathies progressive supranuclear palsy and corticobasal degeneration.[150][151] Other conditions that may present similarly to PD include vascular parkinsonism, Alzheimer's disease, and frontotemporal dementia.[152][153] The International Parkinson and Movement Disorder Society has proposed a set of criteria that, unlike the standard Queen's Square Brain Bank Criteria, includes non-exclusionary "red-flag" clinical features that may not suggest Parkinson's.[154] A large number of "red flags" have been proposed and adopted for various conditions that might mimic the symptoms of PD.[155] Diagnostic tests, including gene sequencing, molecular imaging techniques, and assessment of smell may also distinguish PD.[144] MRI is particularly powerful due to several unique features for atypical parkinsonisms.[144] Key distinguishing symptoms and features include:[139][156][157]
ManagementAs of 2024, no disease-modifying therapies exist that reverse or slow neurodegeneration, processes respectively termed neurorestoration and neuroprotection.[121][122] Patients are typically managed with a holistic approach that combines lifestyle modifications with physical therapy.[158] Current pharmacological interventions purely target symptoms, by either increasing endogenous dopamine levels or directly mimicking dopamine's effect on the patient's brain.[159][158] These include dopamine agonists, MAO-B inhibitors, and levodopa: the most widely used and effective drug.[160][158] The optimal time to initiate pharmacological treatment is debated,[161] but initial dopamine agonist and MAO-B inhibitor treatment and later levodopa therapy is common.[162] Invasive procedures such as deep brain stimulation may be used for patients that do not respond to medication.[163][164] MedicationsLevodopa Levodopa (L-DOPA) is the most widely used and the most effective therapy—the gold standard—for Parkinson's treatment.[160] The compound occurs naturally and is the immediate precursor for dopamine synthesis in the dopaminergic neurons of the substantia nigra.[165] Levodopa administration reduces the dopamine deficiency, alleviating parkinsonian symptoms.[166][167] Despite its efficacy, levodopa poses several challenges and has been called the "pharmacologist's nightmare".[168][169] Its metabolism outside the brain by aromatic L-amino acid decarboxylase (AAAD) and catechol-O-methyltransferase (COMT) can cause nausea and vomiting; inhibitors like carbidopa, entacapone, and benserazide are usually taken with levodopa to mitigate these effects.[170][171][note 1] Symptoms may become unresponsive to levodopa, with sudden changes between a state of mobility ("ON time") and immobility ("OFF time").[173] Long-term levodopa use may also induce dyskinesia and motor fluctuations. Although this often causes levodopa use to be delayed to later stages, earlier administration leads to improved motor function and quality of life.[174] Dopamine agonistsDopamine agonists are an alternative or complement for levodopa therapy. They activate dopamine receptors in the striatum, with reduced risk of motor fluctuations and dyskinesia.[175] Ergot dopamine agonists were commonly used, but have been largely replaced with non-ergot compounds due to severe adverse effects like pulmonary fibrosis and cardiovascular issues.[175] Non-ergot agonists are efficacious in both early and late stage Parkinson's,[176] The agonist apomorphine is often used for drug-resistant OFF time in later-stage PD.[176][177] However, after five years of use, impulse control disorders may occur in over 40 percent of PD patients taking dopamine agonists.[161] A problematic, narcotic-like withdrawal effect may occur when agonist use is reduced or stopped.[161][178] Compared to levodopa, dopamine agonists are more likely to cause fatigue, daytime sleepiness, and hallucinations.[178] MAO-B inhibitorsMAO-B inhibitors—such as safinamide, selegiline and rasagiline—increase the amount of dopamine in the basal ganglia by inhibiting the activity of monoamine oxidase B, an enzyme that breaks down dopamine.[179] These compounds mildly alleviate motor symptoms when used as monotherapy but can also be used with levodopa and can be used at any disease stage.[180] When used with levodopa, time spent in the off phase is reduced.[181][182] Selegiline has been shown to delay the need for initial levodopa, suggesting that it might be neuroprotective and slow the progression of the disease.[183] Common side effects are nausea, dizziness, insomnia, sleepiness, and (in selegiline and rasagiline) orthostatic hypotension.[183][184] MAO-Bs are known to increase serotonin and cause a potentially dangerous condition known as serotonin syndrome.[183][185] Other drugsTreatments for non-motor symptoms of PD have not been well studied and many medications are used off-label.[65] A diverse range of symptoms beyond those related to motor function can be treated pharmaceutically.[186] Examples include cholinesterase inhibitors for cognitive impairment and modafinil for excessive daytime sleepiness.[187] Fludrocortisone, midodrine and droxidopa are commonly used off label for orthostatic hypotension related to autonomic dysfunction. Sublingual atropine or botulinum toxin injections may be used off-label for drooling. SSRIs and SNRIs are often used for depression related to PD, but there is a risk of serotonin syndrome with the SSRI or SNRI antidepressants.[65] Doxepin and rasagline may reduce physical fatigue in PD.[188] Other treatments have received government approval, such as the first FDA-approved treatment for PD psychosis, pimavanserin. Although its efficacy is inferior to off-label clozapine, it has significantly fewer side effects.[189] Invasive interventions Surgery for Parkinson's first appeared in the 19th century and by the 1960s had evolved into ablative brain surgery that lesioned the basal ganglia, thalamus or globus pallidus (a pallidotomy).[190] The discovery of L-DOPA for PD treatment caused ablative therapies to largely disappear.[191][192] Ablative surgeries experienced a resurgence in the 1990s but were quickly superseded by newly-developed deep brain stimulation (DBS).[192] Although gamma knife and high-intensity focused ultrasound surgeries have been developed for pallidotomies and thalamotomies, their use remains rare.[193][194] DBS involves the implantation of electrodes called neurostimulators, which sends electrical impulses to specific parts of the brain.[163] DBS for the subthalamic nucleus and globus pallidus interna has high efficacy for up to 2 years, but longterm efficacy is unclear and likely decreases with time.[163] DBS typically targets rigidity and tremor,[195] and is recommended for PD patients who are intolerant or do not respond to medication.[164] Cognitive impairment is the most common exclusion criteria.[196] Rehabilitation Although pharmacological therapies can improve symptoms, patients' autonomy and ability to perform everyday tasks is still reduced by PD. As a result, rehabilitation is often useful. However, the scientific support for any single rehabilitation treatment is limited.[197] Exercise programs are often recommended, with preliminary evidence of efficacy.[198][199][200] Regular physical exercise with or without physical therapy can be beneficial to maintain and improve mobility, flexibility, strength, gait speed, and quality of life.[198] Aerobic, mind-body, and resistance training may be beneficial in alleviating PD-associated depression and anxiety.[200][201] Strength training may increase manual dexterity and strength, facilitating daily tasks that require grasping objects.[202] In improving flexibility and range of motion for people experiencing rigidity, generalized relaxation techniques such as gentle rocking have been found to decrease excessive muscle tension. Other effective techniques to promote relaxation include slow rotational movements of the extremities and trunk, rhythmic initiation, diaphragmatic breathing, and meditation.[203] Deep diaphragmatic breathing may also improve chest-wall mobility and vital capacity decreased by the stooped posture and respiratory dysfunctions of advanced Parkinson's.[204] Rehabilitation techniques targeting gait and the challenges posed by bradykinesia, shuffling, and decreased arm swing include pole walking, treadmill walking, and marching exercises.[205] Speech therapies such as the Lee Silverman voice treatment may reduce the effect of speech disorders associated with PD.[206][207] Occupational therapy is another rehabilitation strategy and can improve quality of life by enabling PD patients to find engaging activities and communal roles, adapt to their living environment, and improving domestic and work abilities.[208] DietParkinson's poses digestive problems like constipation and prolonged emptying of stomach contents, and a balanced diet with periodical nutritional assessments is recommended to avoid weight loss or gain and minimize the consequences of gastrointestinal dysfunction. In particular, a Mediterranean diet is advised and may slow disease progression.[209][210] As it can compete for uptake with amino acids derived from protein, levodopa should be taken 30 minutes before meals to minimize such competition. Low protein diets may also be needed by later stages.[210] As the disease advances, swallowing difficulties often arise. Using thickening agents for liquid intake and an upright posture when eating may be useful; both measures reduce the risk of choking. Gastrostomy can be used to deliver food directly into the stomach.[211][212] Increased water and fiber intake is used to treat constipation.[213] Palliative careAs Parkinson's is incurable, palliative care aims to improve the quality of life for both the patient and family by alleviating the symptoms and stress associated with illness.[214][215][216] Early integration of palliative care into the disease course is recommended, rather than delaying until later stages.[214] Palliative care specialists can help with physical symptoms, emotional factors such as loss of function and jobs, depression, fear, as well as existential concerns.[217] Palliative care team members also help guide patients and families on difficult decisions caused by disease progression, such as wishes for a feeding tube, noninvasive ventilator or tracheostomy, use of cardiopulmonary resuscitation, and entering hospice care.[218][219] Prognosis
As Parkinson's is a heterogeneous condition with multiple etiologies, prognostication can be difficult and prognoses can be highly variable.[220][222] On average, life expectancy is reduced in those with Parkinson's, with younger age of onset resulting in greater life expectancy decreases.[223] Although PD subtype categorization is controversial, the 2017 Parkinson's Progression Markers Initiative study identified three broad scorable subtypes of increasing severity and more rapid progression: mild-motor predominant, intermediate, and diffuse malignant. Mean years of survival post-diagnosis were 20.2, 13.1, and 8.1.[220] Around 30% of Parkinson's patients develop dementia, and is 12 times more likely to occur in elderly patients of those with severe PD.[224] Dementia is less likely to arise in patients with tremor-dominant PD.[225] Parkinson's disease dementia is associated with a reduced quality of life in people with PD and their caregivers, increased mortality, and a higher probability of needing nursing home care.[226] The incidence rate of falls in Parkinson's patients is approximately 45 to 68%, thrice that of healthy individuals, and half of such falls result in serious secondary injuries. Falls increase morbidity and mortality.[227] Around 90% of those with PD develop hypokinetic dysarthria, which worsens with disease progression and can hinder communication.[228] Additionally, over 80% of PD patients develop dysphagia: consequent inhalation of gastric and oropharyngeal secretions can lead to aspiration pneumonia.[229] Aspiration pneumonia is responsible for 70% of deaths in those with PD.[230] Epidemiology As of 2024, Parkinson's is the second most common neurodegenerative disease and the fastest-growing in total number of cases.[231][232] As of 2023, global prevalence was estimated to be 1.51 per 1000.[233] Although it is around 40% more common in men,[234] age is the dominant predeterminant of Parkinson's.[235] Consequently, as global life expectancy has increased, Parkinson's disease prevalence has also risen, with an estimated increase in cases by 74% from 1990 to 2016.[236] The total number is predicted to rise to over 12 million patients by 2040.[237] Some label this a pandemic.[236] This increase may be due to a number of global factors, including prolonged life expectancy, increased industrialisation, and decreased smoking.[236] Although genetics is the sole factor in a minority of cases, most cases of Parkinson's are likely a result of gene-environment interactions: concordance studies with twins have found Parkinson's heritability to be just 30%.[234] The influence of multiple genetic and environmental factors complicates epidemiological efforts.[238] Relative to Europe and North America, disease prevalence is lower in Africa but similar in Latin America.[239] Although China is predicted to have nearly half of the global Parkinson's population by 2030,[240] estimates of prevalence in Asia vary.[239] Potential explanations for these geographic differences include genetic variation, environmental factors, health care access, and life expectancy.[239] Although PD incidence and prevalence may vary by race and ethnicity, significant disparities in care, diagnosis, and study participation limit generalizability and lead to conflicting results.[239][238] Within the United States, high rates of PD have been identified in the Midwest, the South, and agricultural regions of other states: collectively termed the "PD belt".[241] The association between rural residence and Parkinson's has been hypothesized to be caused by environmental factors like herbicides, pesticides, and industrial waste.[241][242] HistoryIn 1877, Jean-Martin Charcot (left) named the disease for James Parkinson, credited as the first to comprehensively describe it. Patient Pierre D. (right) served as the model for William Gowers' widely distributed illustration of Parkinson's disease.[243] In 1817, English physician James Parkinson published the first full medical description of the disease as a neurological syndrome in his monograph An Essay on the Shaking Palsy.[244][245] He presented six clinical cases, including three he had observed on the streets near Hoxton Square in London.[246] Parkinson described three cardinal symptoms: tremor, postural instability and "paralysis" (undistinguished from rigidity or bradykinesia), and speculated that the disease was caused by trauma to the spinal cord.[247][248] There was little discussion or investigation of the "shaking palsy" until 1861, when Frenchman Jean-Martin Charcot—regarded as the father of neurology—began expanding Parkinson's description, adding bradykinesia as one of the four cardinal symptoms.[247][246][248] In 1877, Charcot renamed the disease after Parkinson, as not all patients displayed the tremor suggested by "shaking palsy".[246][248] Subsequent neurologists who made early advances to the understanding of Parkinson's include Armand Trousseau, William Gowers, Samuel Kinnier Wilson, and Wilhelm Erb.[249]  Although Parkinson is typically credited with the first detailed description of PD, many previous texts reference some of the disease's clinical signs.[250] In his essay, Parkinson himself acknowledged partial descriptions by Galen, William Cullen, Johann Juncker, and others.[248] Possible earlier but incomplete descriptions include a Nineteenth Dynasty Egyptian papyrus, the ayurvedic text Charaka Samhita, Ecclesiastes 12:3, and a discussion of tremors by Leonardo da Vinci.[248][251] Multiple traditional Chinese medicine texts may include references to PD, including a discussion in the Yellow Emperor's Internal Classic (c. 425–221 BC) of a disease with symptoms of tremor, stiffness, staring, and stooped posture.[251] In 2009, a systematic description of PD was found in the Hungarian medical text Pax corporis written by Ferenc Pápai Páriz in 1690, some 120 years before Parkinson. Although Páriz correctly described all four cardinal signs, it was only published in Hungarian and was not widely distributed.[252][253] In 1912, Frederic Lewy described microscopic particles in affected brains, later named Lewy bodies.[254] In 1919, Konstantin Tretiakoff reported that the substantia nigra was the main brain structure affected, corroborated by Rolf Hassler in 1938.[255] The underlying changes in dopamine signaling were identified in the 1950s, largely by Arvid Carlsson and Oleh Hornykiewicz.[256] In 1997, Polymeropoulos and colleagues at the NIH discovered the first gene for PD,[257] SNCA, which encodes alpha-synuclein. Alpha-synuclein was in turn found to be the main component of Lewy bodies by Spillantini, Trojanowski, Goedert, and others.[258] Anticholinergics and surgery were the only treatments until the use of levodopa,[259][260] which, although first synthesized by Casimir Funk in 1911,[261] did not enter clinical use until 1967.[262] By the late 1980s, deep brain stimulation introduced by Alim Louis Benabid and colleagues at Grenoble, France, emerged as an additional treatment.[263] Society and culture Social impactFor some people with PD, masked facial expressions and difficulty moderating facial expressions of emotion or recognizing other people's facial expressions can impact social well-being.[264] As the condition progresses, tremor, other motor symptoms, difficulty communicating, or mobility issues may interfere with social engagement, causing individuals with PD to feel isolated.[265] Public perception and awareness of PD symptoms such as shaking, hallucinating, slurring speech, and being off balance is lacking in some countries and can lead to stigma.[266] CostThe economic cost of Parkinson's to both individuals and society is high.[267] Globally, most government health insurance plans do not cover Parkinson's therapies, requiring patients to pay out-of-pocket.[267] Indirect costs include lifetime earnings losses due to premature death, productivity losses, and caregiver burdens.[268] The duration and progessive nature of PD can place a heavy burden on caregivers:[269] family members like spouses dedicate around 22 hours per week to care.[268] In 2010, the total economic burden of Parkinson's across Europe, including indirect and direct medical costs, was estimated to be €13.9 billion (US $14.9 billion) in 2010.[270] The total burden in the United States was estimated to be $51.9 billion in 2017, and is project to surpass $79 billion by 2037.[268] However, as of 2022, no rigorous economic surveys had been performed for low or middle income nations.[271] Regardless, preventative care has been identified as crucial to prevent the rapidly increasing incidence of Parkinson's from overwhelming national health systems.[269] Advocacy The birthday of James Parkinson, 11 April, has been designated as World Parkinson's Day.[272] A red tulip was chosen by international organizations as the symbol of the disease in 2005; it represents the 'James Parkinson' tulip cultivar, registered in 1981 by a Dutch horticulturalist.[273] Advocacy organizations include the National Parkinson Foundation, which has provided more than $180 million in care, research, and support services since 1982,[274] Parkinson's Disease Foundation, which has distributed more than $115 million for research and nearly $50 million for education and advocacy programs since its founding in 1957 by William Black;[275][276] the American Parkinson Disease Association, founded in 1961;[277] and the European Parkinson's Disease Association, founded in 1992.[278] Notable cases In the 21st century, the diagnosis of Parkinson's among notable figures has increased the public's understanding of the disorder.[279] Actor Michael J. Fox was diagnosed with PD at 29 years old,[280] and has used his diagnosis to increase awareness of the disease.[281] To illustrate the effects of the disease, Fox has appeared without medication in television roles and before the United States Congress without medication.[282] The Michael J. Fox Foundation, which he founded in 2000, has raised over $2 billion for Parkinson's research.[283] Boxer Muhammad Ali showed signs of PD when he was 38, but was undiagnosed until he was 42, and has been called the "world's most famous Parkinson's patient". [284] Whether he had PD or parkinsonism related to boxing is unresolved.[285] Cyclist and Olympic medalist Davis Phinney, diagnosed with Parkinson's at 40, started the Davis Phinney Foundation in 2004 to support PD research.[286][287] Several historical figures have been theorized to have had Parkinson's, often framed in the industriousness and inflexibility of the so-called "Parkinsonian personality".[288][289] For instance, English philosopher Thomas Hobbes was diagnosed with "shaking palsy"—assumed to have been Parkinson's—but continued writing works such as Leviathan.[290][291][292] Adolf Hitler is widely believed to have had Parkinson's, and the condition may have influenced his decision making.[293][294][295] Mao Zedong was also reported to have died from the disorder.[296] Clinical research As of 2024, no disease-modifying therapies exist that reverse or slow the progression of Parkinson's.[121][122] Active research directions include the search for new animal models of the disease and development and trial of gene therapy, stem cell transplants, and neuroprotective agents.[297] Improved treatments will likely combine therapeutic strategies to manage symptoms and enhance outcomes.[298] Reliable biomarkers are needed for early diagnosis, and research criteria for their identification have been established.[299][300] Neuroprotective treatmentsAnti-alpha-synuclein drugs that prevent alpha-synuclein oligomerization and aggregation or promote their clearance are under active investigation, and potential therapeutic strategies include small molecules and immunotherapies like vaccines and monoclonal antibodies.[301][302][303] While immunotherapies show promise, their effiacy is often inconsistent.[302] Anti-inflammatory drugs that target NLRP3 and the JAK-STAT signaling pathway offer another potential therapeutic approach.[304] As the gut microbiome in PD is often disrupted and produces toxic compounds, fecal microbiota transplants might restore a healthy microbiome and alleviate various motor and non-motor symptoms.[301] Neurotrophic factors—peptides that enhance the growth, maturation, and survival of neurons—show modest results but require invasive surgical administration. Viral vectors may represent a more feasible delivery platform.[305] Calcium channel blockers may restore the calcium imbalance present in Parkinson's, and are being investigated as a neuroprotective treatment.[306] Other therapies, like deferiprone, may reduce the abnormal accumulation of iron in PD.[306] Cell-based therapiesResearchers at Argonne National Laboratory examine induced pluripotent stem cells (iPSCs) for use in Parkinson's and other diseases: the action potentials of one such iSPC differentiated into a dopaminergic neuron are visible at right. In contrast to other neurodegenerative disorders, many Parkinson's symptoms can be attributed to the loss of a single cell type. Consequently, dopaminergic neuron regeneration is a promising therapeutic approach.[307] Although most initial research sought to generate dopaminergic neuron precursor cells from fetal brain tissue,[308] pluripotent stem cells—particularly induced pluripotent stem cells (iPSCs)—have become an increasingly popular tissue source.[309][310] Both fetal and iPSC-derived DA neurons have been transplanted into patients in clinical trials.[311][312] Although some patients see improvements, the results are highly variable. Adverse effects, such as dyskinesia arising from excess dopamine release by the transplanted tissues, have also been observed.[313][314] Gene therapyGene therapy for Parkinson's seeks to restore the healthy function of dopaminergic neurons in the substantia nigra by delivering genetic material—typically through a viral vector—to these diseased cells.[315][316] This material may deilver a functional, wildtype version of a gene, or knockdown a pathological variants.[317] Experimental gene therapies for PD have aimed to increase the expression of growth factors or enzymes involved in dopamine synthesis, like tyrosine hydroxylase.[318] The one-time delivery of genes circumvents the recurrent invasive administration required to administer some peptides and proteins to the brain.[319] MicroRNAs are an emerging PD gene therapy platform that may serve as an alternative to viral vectors.[320] Notes and referencesNotes
Citations
Works citedBooks
Journal articles
Web sources
News publications
|
||||||||||||||||||||||||||||||||||||||









