|
Retrosynthetic analysisRetrosynthetic analysis is a technique for solving problems in the planning of organic syntheses. This is achieved by transforming a target molecule into simpler precursor structures regardless of any potential reactivity/interaction with reagents. Each precursor material is examined using the same method. This procedure is repeated until simple or commercially available structures are reached. These simpler/commercially available compounds can be used to form a synthesis of the target molecule. E.J. Corey formalized this concept in his book The Logic of Chemical Synthesis.[1][2][3] The power of retrosynthetic analysis becomes evident in the design of a synthesis. The goal of retrosynthetic analysis is a structural simplification. Often, a synthesis will have more than one possible synthetic route. Retrosynthesis is well suited for discovering different synthetic routes and comparing them in a logical and straightforward fashion.[4] A database may be consulted at each stage of the analysis, to determine whether a component already exists in the literature. In that case, no further exploration of that compound would be required. If that compound exists, it can be a jumping point for further steps developed to reach a synthesis. Definitions
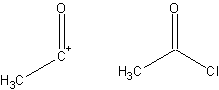
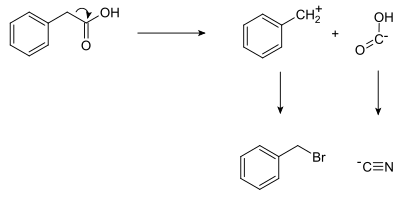
ExampleShown below is a retrosynthetic analysis of phenylacetic acid: In planning the synthesis, two synthons are identified. A nucleophilic "-COOH" group, and an electrophilic "PhCH2+" group. Both synthons do not exist as written; synthetic equivalents corresponding to the synthons are reacted to produce the desired product. In this case, the cyanide anion is the synthetic equivalent for the −COOH synthon, while benzyl bromide is the synthetic equivalent for the benzyl synthon. The synthesis of phenylacetic acid determined by retrosynthetic analysis is thus:
 In fact, phenylacetic acid has been synthesized from benzyl cyanide,[5] itself prepared by the analogous reaction of benzyl bromide with sodium cyanide.[6] StrategiesFunctional group strategiesManipulation of functional groups can lead to significant reductions in molecular complexity. Stereochemical strategiesNumerous chemical targets have distinct stereochemical demands. Stereochemical transformations (such as the Claisen rearrangement and Mitsunobu reaction) can remove or transfer the desired chirality thus simplifying the target. Structure-goal strategiesDirecting a synthesis toward a desirable intermediate can greatly narrow the focus of analysis. This allows bidirectional search techniques. Transform-based strategiesThe application of transformations to retrosynthetic analysis can lead to powerful reductions in molecular complexity. Unfortunately, powerful transform-based retrons are rarely present in complex molecules, and additional synthetic steps are often needed to establish their presence. Topological strategiesThe identification of one or more key bond disconnections may lead to the identification of key substructures or difficult to identify rearrangement transformations in order to identify the key structures.
See alsoReferences
External linksWikiquote has quotations related to Retrosynthetic analysis.
|