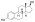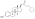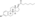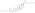Estradiol glucuronide
Names
IUPAC name
3-Hydroxyestra-1,3,5(10)-trien-17β-yl β-D -glucopyranosiduronic acid
Systematic IUPAC name
(2S ,3S ,4S ,5R ,6R )-3,4,5-Trihydroxy-6-{[(1S ,3aS ,3bR ,9bS ,11aS )-7-hydroxy-11a-methyl-2,3,3a,3b,4,5,9b,10,11,11a-decahydro-1H -cyclopenta[a ]phenanthren-1-yl]oxy}oxane-2-carboxylic acid
Other names
E217βG; 17β-Estradiol 17β-D -glucuronide; Estra-1,3,5(10)-triene-3,17β-diol 17β-D -glucuronoside
Identifiers
ChEBI
ChEMBL
ChemSpider
KEGG
InChI=1S/C24H32O8/c1-24-9-8-14-13-5-3-12(25)10-11(13)2-4-15(14)16(24)6-7-17(24)31-23-20(28)18(26)19(27)21(32-23)22(29)30/h3,5,10,14-21,23,25-28H,2,4,6-9H2,1H3,(H,29,30)/t14-,15-,16+,17+,18+,19+,20-,21+,23-,24+/m1/s1
Key: MTKNDAQYHASLID-QXYWQCSFSA-N
C[C@]12CC[C@H]3[C@H]([C@@H]1CC[C@@H]2O[C@H]4[C@@H]([C@H]([C@@H]([C@H](O4)C(=O)O)O)O)O)CCC5=C3C=CC(=C5)O
Properties
C 24 H 32 O 8
Molar mass
−1
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
Chemical compound
Estradiol glucuronide , or estradiol 17β-D -glucuronide , is a conjugated metabolite of estradiol .[ 1] liver by UDP-glucuronyltransferase via attachment of glucuronic acid and is eventually excreted in the urine by the kidneys .[ 1] water solubility than does estradiol.[ 1] [ 1]
When exogenous estradiol is administered orally , it is subject to extensive first-pass metabolism (95%) in the intestines and liver .[ 2] [ 3] absorbed 15% as estrone , 25% as estrone sulfate , 25% as estradiol glucuronide, and 25% as estrone glucuronide .[ 2] estrogen glucuronide conjugates is particularly important with oral estradiol as the percentage of estrogen glucuronide conjugates in circulation is much higher with oral ingestion than with parenteral estradiol.[ 2] elimination half-life of oral estradiol.[ 2] pharmacokinetics of estradiol,[ 2] [ 4] intravenous injection its elimination half-life is only about 1 to 2 hours.[ 5]
Approximately 7% of estradiol is excreted in the urine as estradiol glucuronide.[ 6]
Estradiol glucuronide is transported into prostate gland , testis , and breast cells by OATP1A2 , OATP1B1 , OATP1B3 , OATP1C1 , and OATP3A1 .[ 7] ABC transporters MRP2 , MRP3 , MRP4 , and BCRP , as well as several other transporters, have been found to transport estradiol glucuronide out of cells.[ 7] [ 8]
The circulating concentrations of estrogen glucuronides are generally more than 10-fold lower than those of estrone sulfate , the most abundant estrogen conjugate in the circulation.[ 8]
Estradiol glucuronide has been identified as an agonist of the G protein-coupled estrogen receptor (GPER), a membrane estrogen receptor .[ 9] cholestasis .[ 9]
Estrogen glucuronides can be deglucuronidated into the corresponding free estrogens by β-glucuronidase in tissues that express this enzyme , such as the mammary gland .[ 10] [ 10]
Estradiol glucuronide shows about 300-fold lower potency in activating the estrogen receptors relative to estradiol in vitro [ 11]
The positional isomer of estradiol glucuronide, estradiol 3-glucuronide , also occurs as a major endogenous metabolite of estradiol, circulating at two-thirds of the levels of estrone sulfate when it reaches its maximal concentrations just before ovulation and during the peak in estradiol levels that occurs at this time.[ 12]
Structural properties of selected estradiol esters
Estrogen
Structure
Ester(s)
Relativemol. weight
RelativeE2 contentb
log Pc
Position(s)
Moiet(ies)
Type
Lengtha
Estradiol –
–
–
–
1.00
1.00
4.0
Estradiol acetate C3
Ethanoic acid Straight-chain fatty acid
2
1.15
0.87
4.2
Estradiol benzoate C3
Benzoic acid Aromatic fatty acid
– (~4–5)
1.38
0.72
4.7
Estradiol dipropionate C3, C17β
Propanoic acid (×2)Straight-chain fatty acid
3 (×2)
1.41
0.71
4.9
Estradiol valerate C17β
Pentanoic acid Straight-chain fatty acid
5
1.31
0.76
5.6–6.3
Estradiol benzoate butyrate C3, C17β
Benzoic acid , butyric acid Mixed fatty acid
– (~6, 2)
1.64
0.61
6.3
Estradiol cypionate C17β
Cyclopentylpropanoic acid Cyclic fatty acid
– (~6)
1.46
0.69
6.9
Estradiol enanthate C17β
Heptanoic acid Straight-chain fatty acid
7
1.41
0.71
6.7–7.3
Estradiol dienanthate C3, C17β
Heptanoic acid (×2)Straight-chain fatty acid
7 (×2)
1.82
0.55
8.1–10.4
Estradiol undecylate C17β
Undecanoic acid Straight-chain fatty acid
11
1.62
0.62
9.2–9.8
Estradiol stearate C17β
Octadecanoic acid Straight-chain fatty acid
18
1.98
0.51
12.2–12.4
Estradiol distearate C3, C17β
Octadecanoic acid (×2)Straight-chain fatty acid
18 (×2)
2.96
0.34
20.2
Estradiol sulfate C3
Sulfuric acid Water-soluble conjugate
–
1.29
0.77
0.3–3.8
Estradiol glucuronide
C17β
Glucuronic acid Water-soluble conjugate
–
1.65
0.61
2.1–2.7
Estramustine phosphate d C3, C17β
Normustine , phosphoric acid Water-soluble conjugate
–
1.91
0.52
2.9–5.0
Polyestradiol phosphate e C3–C17β
Phosphoric acid Water-soluble conjugate
–
1.23f
0.81f
2.9g
Footnotes: a = Length of ester in carbon atoms for straight-chain fatty acids or approximate length of ester in carbon atoms for aromatic or cyclic fatty acids. b = Relative estradiol content by weight (i.e., relative estrogenic exposure). c = Experimental or predicted octanol/water partition coefficient (i.e., lipophilicity /hydrophobicity ). Retrieved from PubChem , ChemSpider , and DrugBank . d = Also known as estradiol normustine phosphate . e = Polymer of estradiol phosphate (~13 repeat units ). f = Relative molecular weight or estradiol content per repeat unit. g = log P of repeat unit (i.e., estradiol phosphate). Sources: See individual articles.
See also
References
^ a b c d "Human Metabolome Database: Showing metabocard for 17-beta-Estradiol glucuronide (HMDB0010317)" .^ a b c d e Michael Oettel; Ekkehard Schillinger (6 December 2012). Estrogens and Antiestrogens II: Pharmacology and Clinical Application of Estrogens and Antiestrogen ISBN 978-3-642-60107-1 ^ M. Notelovitz; P.A. van Keep (6 December 2012). The Climacteric in Perspective: Proceedings of the Fourth International Congress on the Menopause, held at Lake Buena Vista, Florida, October 28–November 2, 1984 ISBN 978-94-009-4145-8 ^ Stanczyk, Frank Z.; Archer, David F.; Bhavnani, Bhagu R. (2013). "Ethinyl estradiol and 17β-estradiol in combined oral contraceptives: pharmacokinetics, pharmacodynamics and risk assessment". Contraception . 87 (6): 706–727. doi :10.1016/j.contraception.2012.12.011 . ISSN 0010-7824 . PMID 23375353 . ^ Düsterberg B, Nishino Y (1982). "Pharmacokinetic and pharmacological features of oestradiol valerate". Maturitas . 4 (4): 315–24. doi :10.1016/0378-5122(82)90064-0 . PMID 7169965 . ^ Kelly Smith; Daniel M. Riche; Nickole Henyan (15 April 2010). Clinical Drug Data, 11th Edition ISBN 978-0-07-162686-6 ^ a b Mueller JW, Gilligan LC, Idkowiak J, Arlt W, Foster PA (October 2015). "The Regulation of Steroid Action by Sulfation and Desulfation" . Endocr. Rev . 36 (5): 526–63. doi :10.1210/er.2015-1036 . PMC 4591525 PMID 26213785 . ^ a b Järvinen E, Deng F, Kidron H, Finel M (April 2018). "Efflux transport of estrogen glucuronides by human MRP2, MRP3, MRP4 and BCRP". J. Steroid Biochem. Mol. Biol . 178 : 99–107. doi :10.1016/j.jsbmb.2017.11.007 . hdl :10138/321530 PMID 29175180 . S2CID 3678002 . ^ a b Zucchetti AE, Barosso IR, Boaglio AC, Basiglio CL, Miszczuk G, Larocca MC, Ruiz ML, Davio CA, Roma MG, Crocenzi FA, Pozzi EJ (March 2014). "G-protein-coupled receptor 30/adenylyl cyclase/protein kinase A pathway is involved in estradiol 17β-D-glucuronide-induced cholestasis". Hepatology . 59 (3): 1016–29. doi :10.1002/hep.26752 . hdl :2133/10484 PMID 24115158 . S2CID 20568614 . ^ a b Zhu BT, Conney AH (January 1998). "Functional role of estrogen metabolism in target cells: review and perspectives" . Carcinogenesis . 19 (1): 1–27. doi :10.1093/carcin/19.1.1 PMID 9472688 . ^ Coldham NG, Dave M, Sivapathasundaram S, McDonnell DP, Connor C, Sauer MJ (July 1997). "Evaluation of a recombinant yeast cell estrogen screening assay" . Environ. Health Perspect . 105 (7): 734–42. doi :10.1289/ehp.97105734 . PMC 1470103 PMID 9294720 . ^ F. A. Kincl; J. R. Pasqualini (22 October 2013). Hormones and the Fetus: Volume 1: Production, Concentration and Metabolism During Pregnancy ISBN 978-1-4832-8538-2
External links
ER Tooltip Estrogen receptor
Agonists
Steroidal: 2-Hydroxyestradiol 2-Hydroxyestrone 3-Methyl-19-methyleneandrosta-3,5-dien-17β-ol 3α-Androstanediol 3α,5α-Dihydrolevonorgestrel 3β,5α-Dihydrolevonorgestrel 3α-Hydroxytibolone 3β-Hydroxytibolone 3β-Androstanediol 4-Androstenediol 4-Androstenedione 4-Fluoroestradiol 4-Hydroxyestradiol 4-Hydroxyestrone 4-Methoxyestradiol 4-Methoxyestrone 5-Androstenediol 7-Oxo-DHEA 7α-Hydroxy-DHEA 7α-Methylestradiol 7β-Hydroxyepiandrosterone 8,9-Dehydroestradiol 8,9-Dehydroestrone 8β-VE2 10β,17β-Dihydroxyestra-1,4-dien-3-one (DHED) 11β-Chloromethylestradiol 11β-Methoxyestradiol 15α-Hydroxyestradiol 16-Ketoestradiol 16-Ketoestrone 16α-Fluoroestradiol 16α-Hydroxy-DHEA 16α-Hydroxyestrone 16α-Iodoestradiol 16α-LE2 16β-Hydroxyestrone 16β,17α-Epiestriol (16β-hydroxy-17α-estradiol) 17α-Estradiol (alfatradiol )17α-Dihydroequilenin 17α-Dihydroequilin 17α-Epiestriol (16α-hydroxy-17α-estradiol) 17α-Ethynyl-3α-androstanediol 17α-Ethynyl-3β-androstanediol 17β-Dihydroequilenin 17β-Dihydroequilin 17β-Methyl-17α-dihydroequilenin Abiraterone Abiraterone acetate Alestramustine Almestrone Anabolic steroids (e.g., testosterone and esters , methyltestosterone , metandienone (methandrostenolone) , nandrolone and esters , many others; via estrogenic metabolites)Atrimustine Bolandiol Bolandiol dipropionate Butolame Clomestrone Cloxestradiol
Conjugated estriol Conjugated estrogens Cyclodiol Cyclotriol DHEA DHEA-S ent -EstradiolEpiestriol (16β-epiestriol, 16β-hydroxy-17β-estradiol) Epimestrol Equilenin Equilin ERA-63 (ORG-37663) Esterified estrogens Estetrol Estradiol
Estramustine Estramustine phosphate Estrapronicate Estrazinol Estriol
Estrofurate Estrogenic substances Estromustine Estrone
Etamestrol (eptamestrol) Ethinylandrostenediol
Ethinylestradiol
Ethinylestriol Ethylestradiol Etynodiol Etynodiol diacetate Hexolame Hippulin Hydroxyestrone diacetate Lynestrenol Lynestrenol phenylpropionate Mestranol Methylestradiol Moxestrol Mytatrienediol Nilestriol Norethisterone Noretynodrel Orestrate Pentolame Prodiame Prolame Promestriene RU-16117 Quinestradol Quinestrol Tibolone Xenoestrogens: Anise -related (e.g., anethole , anol , dianethole , dianol , photoanethole )Chalconoids (e.g., isoliquiritigenin , phloretin , phlorizin (phloridzin) , wedelolactone )Coumestans (e.g., coumestrol , psoralidin )Flavonoids (incl. 7,8-DHF , 8-prenylnaringenin , apigenin , baicalein , baicalin , biochanin A , calycosin , catechin , daidzein , daidzin , ECG , EGCG , epicatechin , equol , formononetin , glabrene , glabridin , genistein , genistin , glycitein , kaempferol , liquiritigenin , mirificin , myricetin , naringenin , penduletin , pinocembrin , prunetin , puerarin , quercetin , tectoridin , tectorigenin )Lavender oil Lignans (e.g., enterodiol , enterolactone , nyasol (cis -hinokiresinol) )Metalloestrogens (e.g., cadmium )Pesticides (e.g., alternariol , dieldrin , endosulfan , fenarimol , HPTE , methiocarb , methoxychlor , triclocarban , triclosan )Phytosteroids (e.g., digitoxin (digitalis ), diosgenin , guggulsterone )Phytosterols (e.g., β-sitosterol , campesterol , stigmasterol )Resorcylic acid lactones (e.g., zearalanone , α-zearalenol , β-zearalenol , zearalenone , zeranol (α-zearalanol) , taleranol (teranol, β-zearalanol) )Steroid -like (e.g., deoxymiroestrol , miroestrol )Stilbenoids (e.g., resveratrol , rhaponticin )Synthetic xenoestrogens (e.g., alkylphenols , bisphenols (e.g., BPA , BPF , BPS ), DDT , parabens , PBBs , PHBA , phthalates , PCBs )Others (e.g., agnuside , rotundifuran ) MixedSERMs Tooltip Selective estrogen receptor modulators ) Antagonists
Coregulator-binding modulators: ERX-11
GPER Tooltip G protein-coupled estrogen receptor
Agonists Antagonists Unknown